メモリーT細胞の培養に係る特許
当社の培養法は、前会長である関根博士が国立がんセンター在籍時に開発したものです。当社はこの培養技術を海外に導出し、韓国では12,000人に投与され、累計で80,000回投与分のT細胞の培養を行いました。また、メモリーT細胞の培養に係る特許(特許第6142142号)技術を保有しており、当社の培養の基盤となっております。

関根法
 関根博士は、米国のローゼンバーグが開発した養子免疫療法に刺激を受け、免疫によるがん治療研究に着手しました。彼は特に、ローゼンバーグらが研究していたTIL(腫瘍浸潤リンパ球)の臨床応用における増殖率の限界に着目しました 。ローゼンバーグらの手法ではリンパ球の増殖率は10〜100倍程度に過ぎず、治療に必要な大量のリンパ球を確保できないことが課題でした。
関根博士は、米国のローゼンバーグが開発した養子免疫療法に刺激を受け、免疫によるがん治療研究に着手しました。彼は特に、ローゼンバーグらが研究していたTIL(腫瘍浸潤リンパ球)の臨床応用における増殖率の限界に着目しました 。ローゼンバーグらの手法ではリンパ球の増殖率は10〜100倍程度に過ぎず、治療に必要な大量のリンパ球を確保できないことが課題でした。関根は、1985年に解明されたT細胞受容体(TCR)の構造と、TCRに抗体が結合することでT細胞が活性化・増殖し、IL2受容体を発現するという知見に着想を得ました 。彼は、がん治療や診断薬として研究が進められていたモノクローナル抗体に着目し、特にT細胞の免疫機能を失わせる作用を持つCD3抗体「OKT3」を、T細胞の大量増殖に利用するという大胆なアイデアを考案しました。
このアイデアに基づき実験を行った結果、T細胞は2週間で最大5万倍にまで増殖するという驚くべき成果が得られました。これはIL2単独による増殖率とは桁違いの成果であり、増殖後のT細胞の殺腫瘍細胞活性も維持されていることが確認されました。
この成功により確立されたのが、正式名称「固相化CD3抗体によるTリンパ球培養法」、通称「関根法」です。この培養法は、OKT3を培養容器の底に固着させる「固相化」という手法を用いる第一段階の「活性化培養」と、OKT3を含まない容器で増殖を続ける第二段階の「増幅培養」から構成されます。関根は培養に必要な器具や資材の開発・改良も進め、1988年いっぱいでこの大量培養法を完成させました。
関根は完成後すぐの1989年初頭から、この培養法マニュアルを全国の医療機関30施設以上に配布し始めました。リンパ球細胞を数千倍、数万倍に増やすという常識を超えた成果であったため、当初は学会誌の編集委員会からがん化の可能性を指摘されるほどでしたが、関根は改めて証明実験を行い、1993年にその論文掲載を認めさせました。関根法は、その後のリンパ球療法の臨床研究の基盤となり、日本における養子免疫療法の進展に大きく貢献しました。
細胞の凍結・解凍技術
 株式会社GCリンフォテックは、細胞の凍結保存および解凍に関する高度な技術を有しており、自社で培養したヒトリンパ球や間葉系幹細胞(MSC)などの細胞を凍結保存しています。独自に確立した解凍技術により、解凍後も高い生存率と機能性を維持できることから、これらの技術を通じて、長年にわたり多くの医療機関へ高品質な細胞を安定的に提供しています。
株式会社GCリンフォテックは、細胞の凍結保存および解凍に関する高度な技術を有しており、自社で培養したヒトリンパ球や間葉系幹細胞(MSC)などの細胞を凍結保存しています。独自に確立した解凍技術により、解凍後も高い生存率と機能性を維持できることから、これらの技術を通じて、長年にわたり多くの医療機関へ高品質な細胞を安定的に提供しています。また、当社では細胞凍結保存液「バンバンカー」シリーズを自社で開発・製造しており、20年以上にわたって販売を継続しています。これらの製品は、日本国内のみならず、世界各国においても高い評価をいただいています。さらに、一部の製品については、再生医療等製品の原材料として使用可能であることを証明する「再生医療等製品材料適格性確認書」を取得しており、再生医療分野での利用にも対応しています。
製品の製造は、GMP(適正製造基準)に準拠した専用施設で行っており、加えて、国際的な品質マネジメントシステムであるISO 9001の認証も取得しています。これにより、品質と安全性に優れた製品を安定して提供できる体制を整えています。
細胞の凍結保存や解凍に関するご相談がございましたら、どうぞお気軽にGCリンフォテックまでお問い合わせください。
NK細胞培養に関する技術
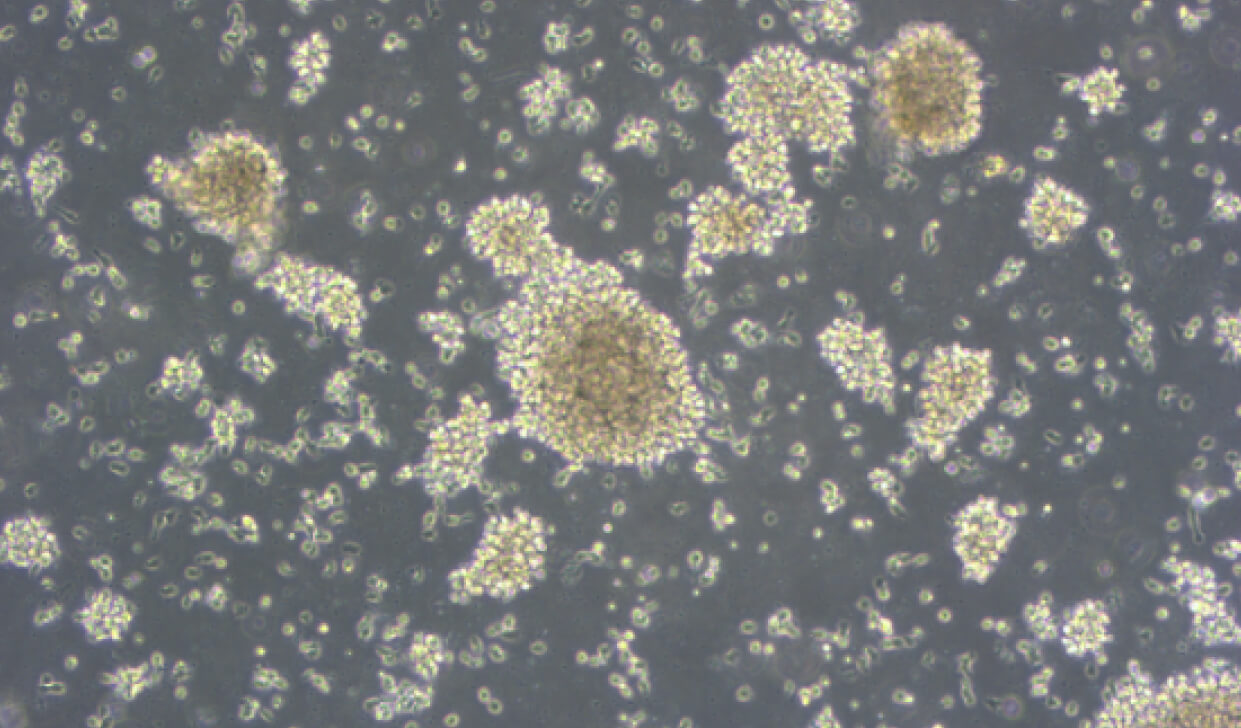 当社は、ナチュラルキラー(NK)細胞の製造において、独自に開発した培地および培養法を用い、高活性かつ安定的な細胞供給を実現しています。培養には、動物由来成分を一切含まない、ヒト由来アルブミンを使用した低血清培地を採用しており、安全性と再現性の高い細胞製造環境を提供しています。
当社は、ナチュラルキラー(NK)細胞の製造において、独自に開発した培地および培養法を用い、高活性かつ安定的な細胞供給を実現しています。培養には、動物由来成分を一切含まない、ヒト由来アルブミンを使用した低血清培地を採用しており、安全性と再現性の高い細胞製造環境を提供しています。また近年の解析により、当社の培養条件下ではNK細胞のみならず、サイトカイン誘導キラー(CIK)細胞も含まれる集団が得られていることが明らかになりました。これにより、より多様な細胞免疫活性を持つ製品の提供が可能となり、がん免疫療法をはじめとする応用範囲がさらに広がっています。
さらに、当社の培地の一部は、再生医療等製品の原材料として使用可能な「材料適格性確認書」を取得しており、臨床応用にも対応しています。
特長:
• 独自開発の培地・培養法によるNK/CIK細胞の効率的増殖
• 動物由来成分フリー・ヒト由来アルブミン配合の低血清培地
• 材料適格性確認済みの培地による高い安全性と信頼性
• GMP準拠の製造設備と徹底した品質管理体制
当社は今後も、NK細胞およびCIK細胞の機能を最大限に活かした免疫細胞技術の開発を通じて、がんや再生医療の分野に貢献してまいります。
活性化自己リンパ球療法のエビデンス
国立がん研究センターの無作為比較試験
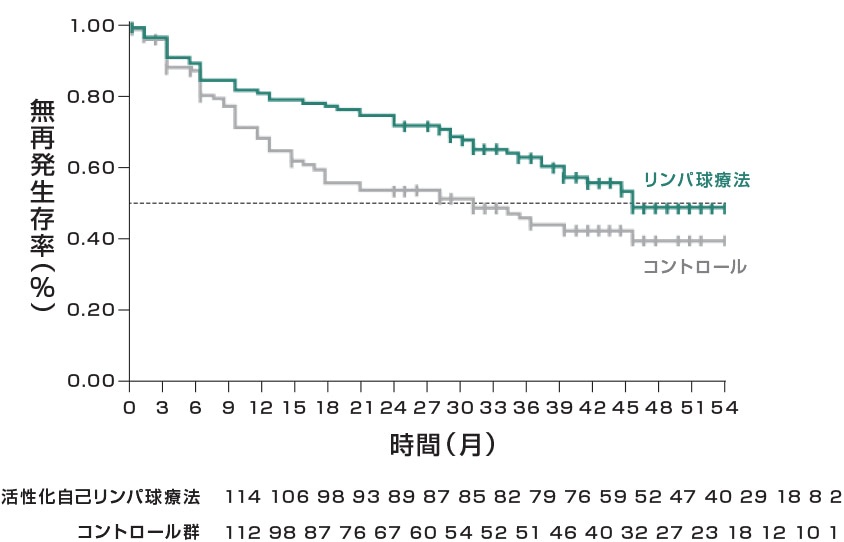
国立がん研究センターにおいて肝がんの手術後の再発予防を目的に無作為化比較試験を実施。
免疫細胞療法(CAR-T細胞療法以外)として日本人を対象に有意差が認められています。
無再発生存率
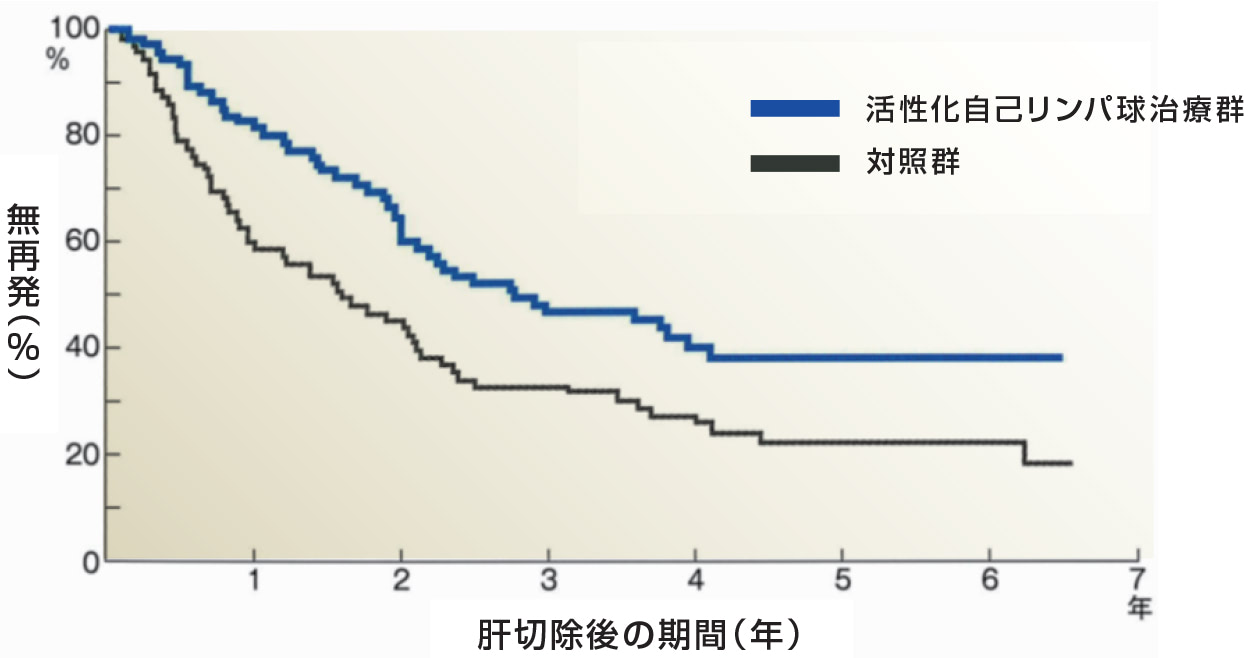
活性化自己リンパ球(平均細胞数710億個/CD3及びHLA-DR細胞78%)6か月で5回の投与により肝細胞癌の無再発生存期間が有意に延長されました。
※Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellularcarcinoma: a randomised trial: THE LANCET p802-807 September 02, 2000
CIK細胞
サイトカイン誘導キラー(CIK)細胞
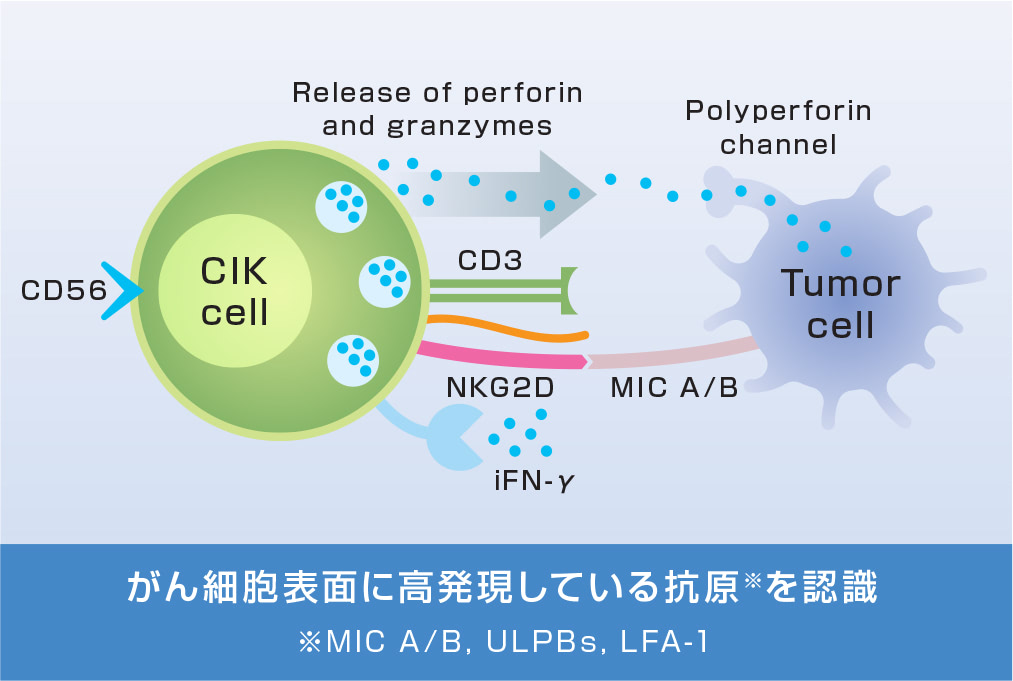
細胞障害性Tリンパ球(CTL)
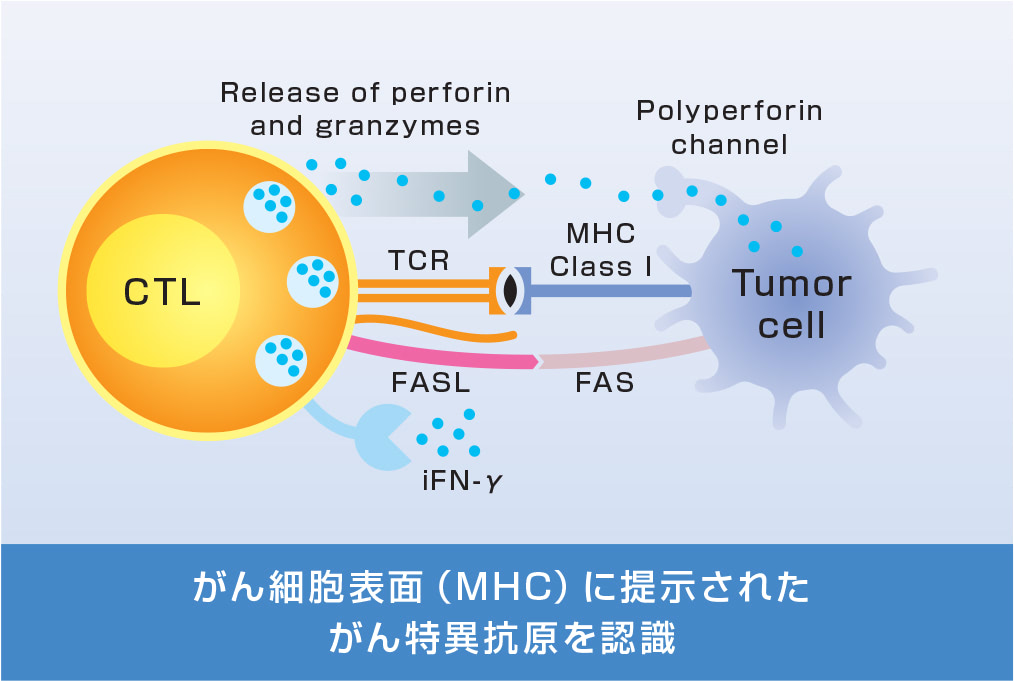
| CIK細胞 サイトカイン誘導キラー細胞 |
CTL細胞 細胞障害性T細胞 |
|
|---|---|---|
| 構成 | CD3+/CD56+ | CD3+/CD8+ |
| 標的認識方法 | MHC非依存的 | 抗原特異的 |
| がん細胞攻撃機序 | パーフォリン・グランザイム経路 FAS-FASL経路 ADCC活性 |
パーフォリン・グランザイム経路 FAS-FASL経路 |
| TCRの有無と働き | あり(抗原認識能はなし) | あり(TCRを介して抗原を認識) |
| MHCクラスI認識能 | MHCクラスI低下細胞を攻撃 | MHCクラスIに結合 |
※以下、参考文献より作図
Wang X, et al. Cell Immunol. 2014;287(1):18-22.
Jiang J, et al. J Transl Med. 2013;11:83.
Jiang J, et al. J Transl Med. 2013;11:83.
【参考】韓国臨床試験データ
2005年に韓国へ細胞培養技術をライセンスアウトし、この技術を使用した「Immuncell-LC」が2007年に韓国FDAから医薬品として承認されました。同商品は2018年にアメリカFDAの希少疾病用医薬品にも指定されました。
韓国の医療機関16施設、肝細胞がん患者230例にて無作為化比較試験を実施。
承認取得を目的とした最もエビデンスレベルが高いランダム化比較試験です。
無再発生存期間
全生存率
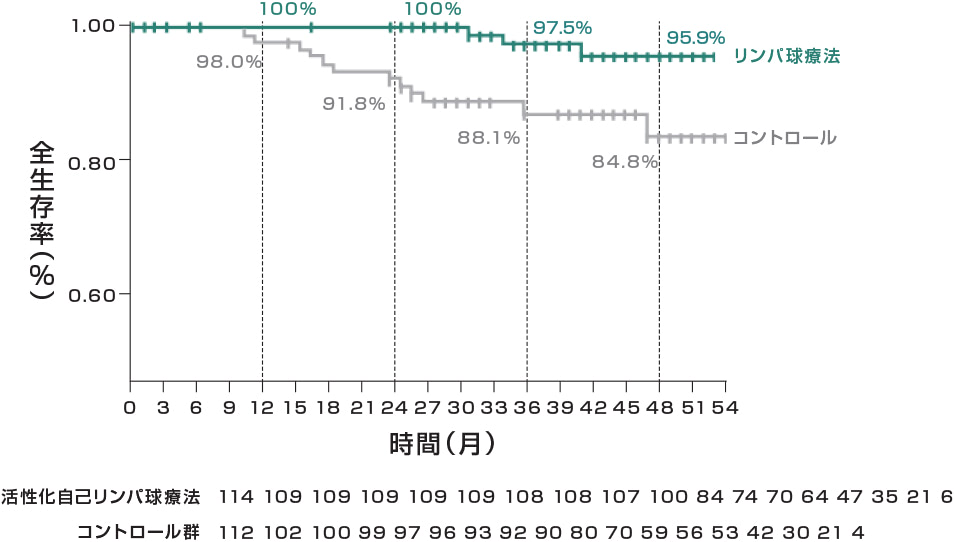
活性化CIK細胞(総細胞数の平均64億個)60週間で16回の投与により、
肝細胞がんの再発リスク37%減少、死亡リスク79%減少することが示されました。
※RFS:無再発生存率CSS:がん特異的生存率
※Gastroenterology.2015;148(7): 1383-1391.e6.
主な副作用は、発熱(11%)疲労(10%)悪寒(9%)で、関連するグレード3以上の
有害事象の発現はありませんでした。
| AES:Safety population | ||||||||
|---|---|---|---|---|---|---|---|---|
| All AE | Related AE | Control (n=115) All AE | Pvalue | |||||
| Any grade | Grade 3-4 | Any grade | Grade 3-4 | Any grade | Grade 3-4 | Any grade | Grade 3-4 | |
| 全体 | 71 (62%) | 7 (6%) | 40 (35%) | 0 | 47 (41%) | 4 (4%) | 0.002 | 0.354 |
| 嘔吐 | 3 (3%) | 0 | 1 (1%) | 0 | 3 (3%) | 1 (1%) | 1.00 | 1.00 |
| 悪寒 | 10 (9%) | 0 | 9 (8%) | 0 | 0 | 0 | 0.001 | NA |
| 倦怠感 | 11 (10%) | 0 | 3 (3%) | 0 | 3 (3%) | 0 | 0.03 | NA |
| 発熱 | 13 (11%) | 0 | 10 (9%) | 0 | 0 | 0 | <0.001 | NA |
| 上気道 感染 |
7 (6%) | 0 | 0 | 0 | 3 (3%) | 0 | 0.20 | NA |
| 頭痛 | 3 (3%) | 0 | 2 (2%) | 0 | 1 (1%) | 1 (1%) | 0.62 | 1.00 |
| 湿性咳嗽 | 6 (5%) | 0 | 0 | 0 | 0 | 0 | 0.03 | NA |
CN-square test: Fisher exact test.
※Immuncell-LC: GC Lymphotecがライセンスアウトした活性化自己リンパ球療法
韓国第Ⅲ相試験の結果は、肝癌治療ガイドライン、NCCN肝細胞癌ガイドライン、EASL臨床実践ガイドラインに記載されています。
また、5年長期追跡調査や9年長期追跡調査において、CIK群はOSの改善という一貫した傾向を示しました。
※当社製品のデータではございません
【参考】抗PD-1交代との併用
進行非小細胞肺癌患者(n=18)に対して、CIK療法と抗PD-1抗体の併用と抗PD-1抗体単独PembrolizumabとCIK細胞の併用は高い奏効率(ORR42.86%)を示しました。
無増悪生存期間
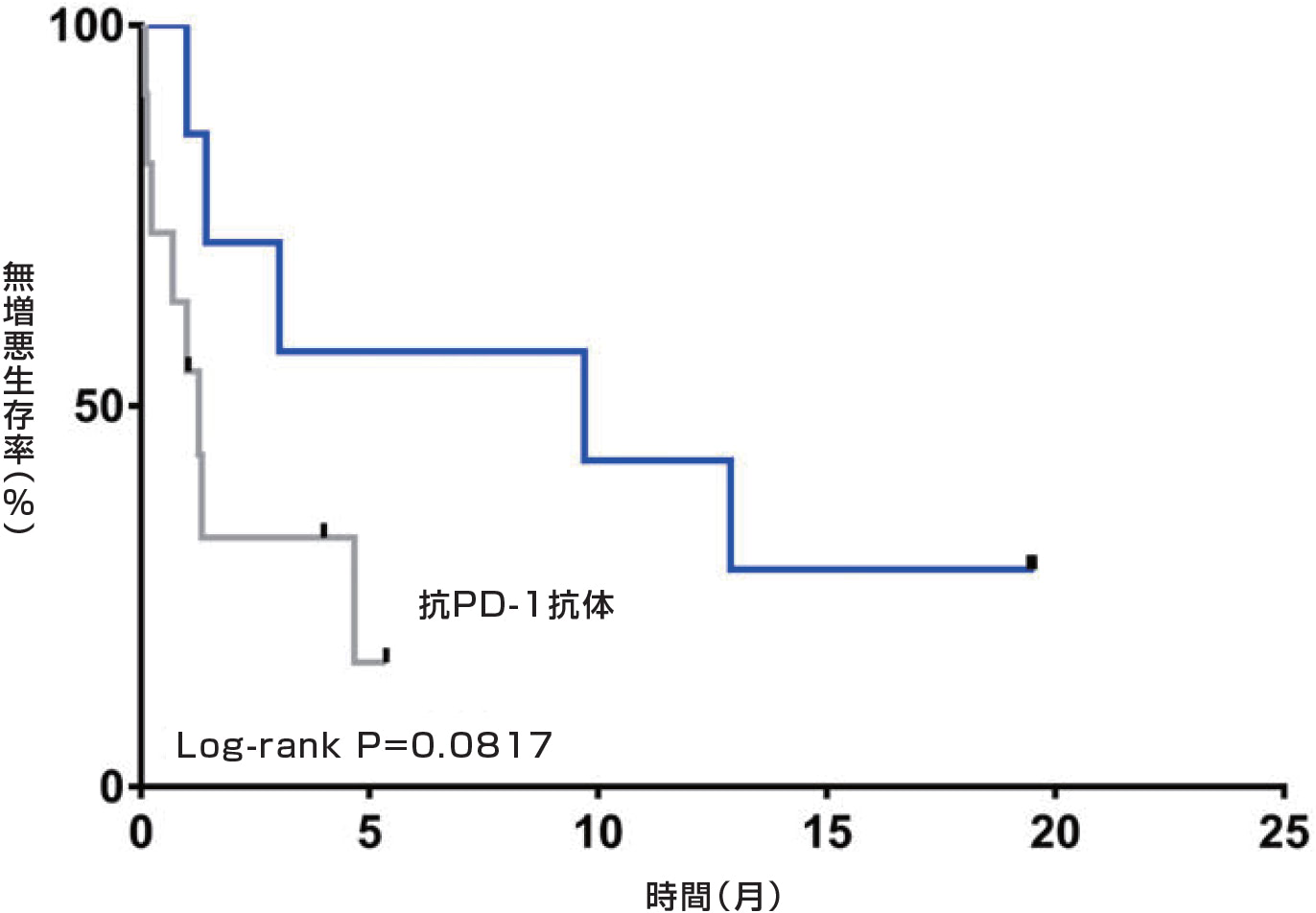
Han Y, et al. Thorac Cancer.2021; 12(2): 145-152.
CIK療法とPD-1/PD-L1阻害剤の併用により、肺がん治療の効果向上が期待されています。
この組み合わせは、CIK細胞の抗腫瘍活性を強化し、腫瘍微小環境の免疫抑制を解除する可能性があります。
本予備研究では、進行非小細胞肺癌(NSCLC)患者18名を対象に、PD-1阻害抗体ペンブロリズマブまたはニボルマブを、自家CIK細胞輸注の有無にかかわらず併用投与した場合の安全性および免疫機能への有効性を検討した。これらの患者から末梢血単核細胞を単離し、フローサイトメトリーを用いてPD-1などの細胞表面分子の発現レベルを検出し、併用療法の有効性を評価した。
OS中央値は28.4ヶ月であったのに対し、併用群のOS中央値は21.7ヶ月であった。PFSの中央値は、併用群で9.7ヶ月、PD-1阻害抗体療法単独群で1.3ヶ月であった。併用群では生存期間の有意な延長は認められなかったものの、PD-1阻害抗体療法単独群と比較して死亡例は少なかった。
※当社製品のデータではございません
【参考】NK細胞との併用
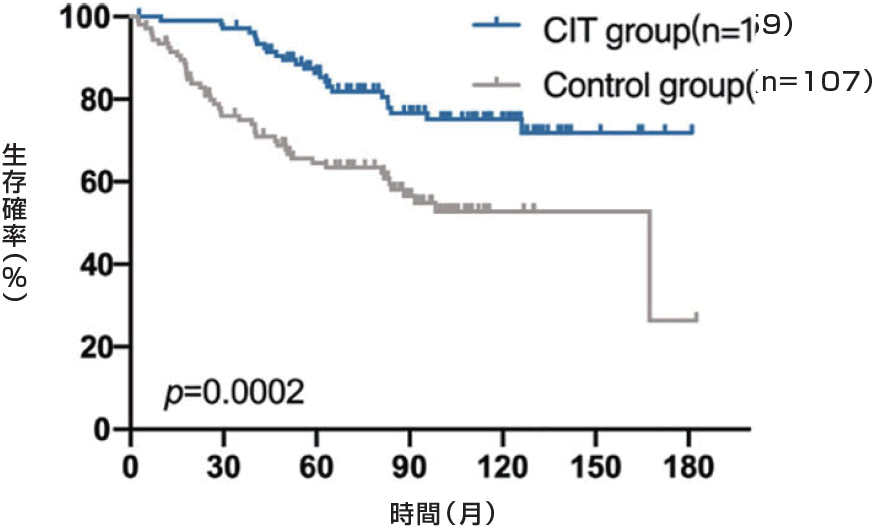
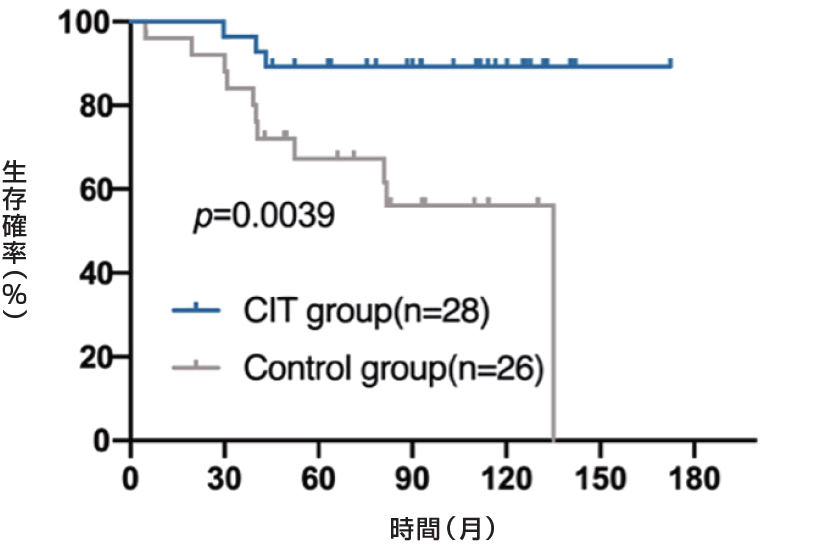
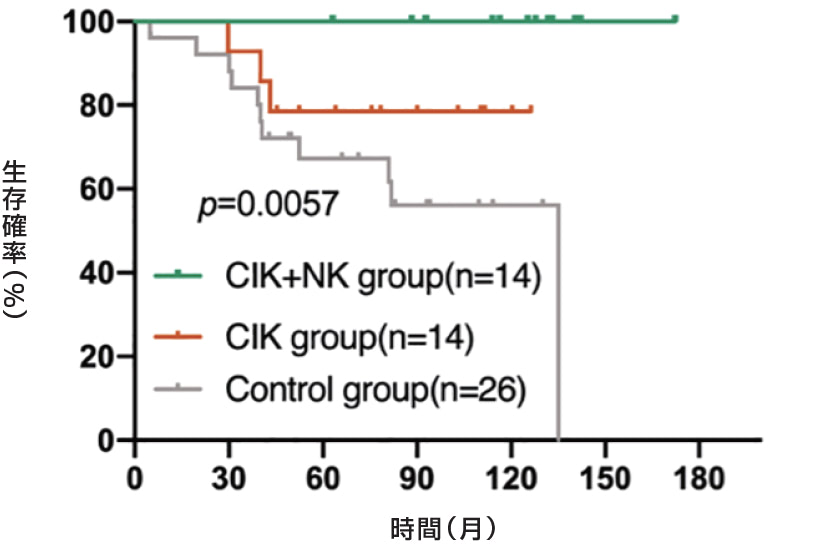
乳房切除後の乳がん患者に対してCIK細胞とNK細胞を交互に投与し、生存率が向上しました。
A
B
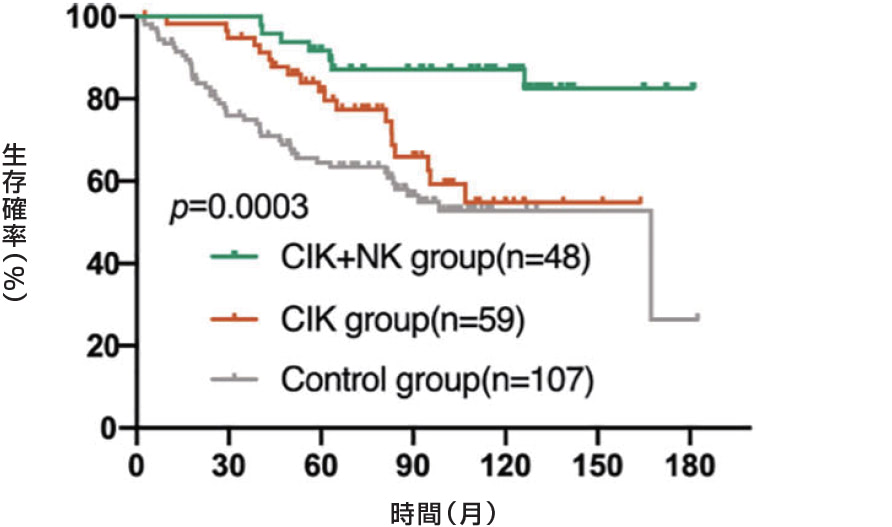
TNBC(トリプルネガティブ)の乳がん患者の生存率が向上しました。
A: TNBC subgroup
B: NO-TNBC subgroup
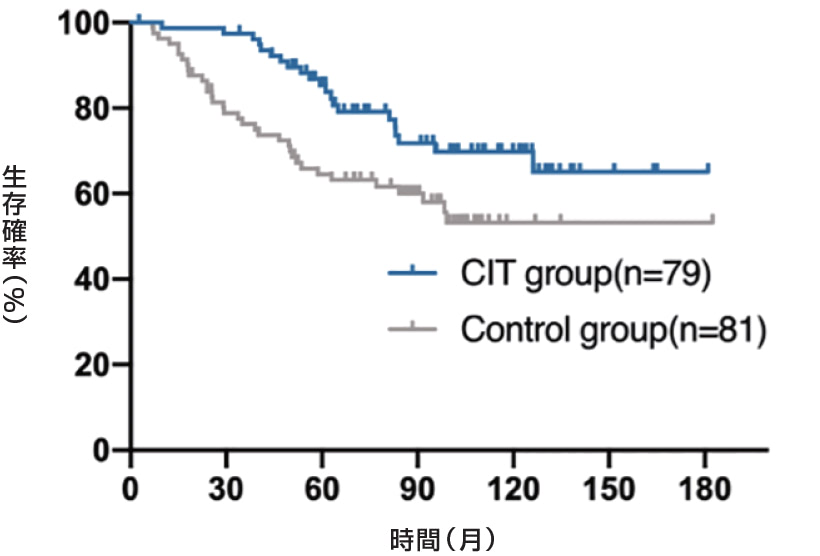
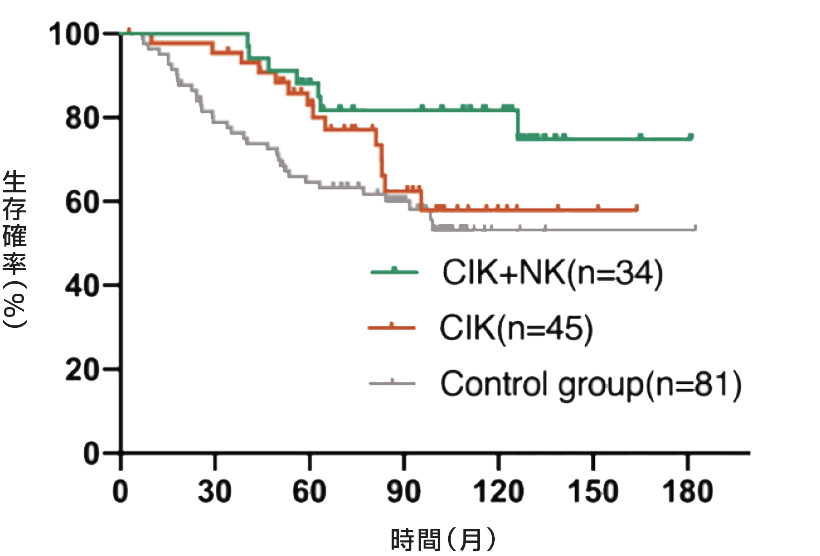
※Xinyi Yang, et al. cancer.Front Immunol. 2022 Nov 10:13:974487.
乳房切除後乳がん患者における術後細胞免疫療法(CIT)について検討しました。乳房切除後乳がん患者214名を登録し、うち107名を対照群(化学療法、放射線療法、内分泌療法を実施)、残りの107名をCIT群(化学療法、放射線療法、内分泌療法を実施し、その後免疫細胞を注入)としました。この214名のうち、54名がTNBCで、対照群26名とCIT群28名でした。
※当社製品のデータではございません
当社保有の特許リスト
• メモリーT細胞を主成分とするリンパ球細胞群の製造方法(特許第6142142号)
• マイコプラズマを検出する方法(特許第6704565号)
• CO2インキュベータのオゾン滅菌装置(特許第5278861号)
• 内因性DHEA-Sを増加させるための製剤およびその製造方法、ならびに内因性DHEA-Sを増加させるための方法(特許第5561845号)
• CO2インキュベータのオゾン滅菌方法(特許第5209415号)
• 遠心分離機を組み込んだ安全キャビネット及びバイオクリーンベンチ(特許第4238861号)
• 内容物の温度の調整方法、及び、内容物の調温容器(特許第6671582号)
• ヒトリンパ球細胞培養用無血清培地(特許第7144872号)